An lis is used to support workflows in the laboratory as well as the repository to store laboratory data while supporting the laboratory mission.
Lis laboratory information system open source.
A laboratory information system lis is a computer based information management system creat ed specifically for laboratories.
Furthermore it generates an unlimited number of report formats and types for each physician and pathologist.
An entry level laboratory information system which allows to eliminate paper media from all stages of the laboratory process.
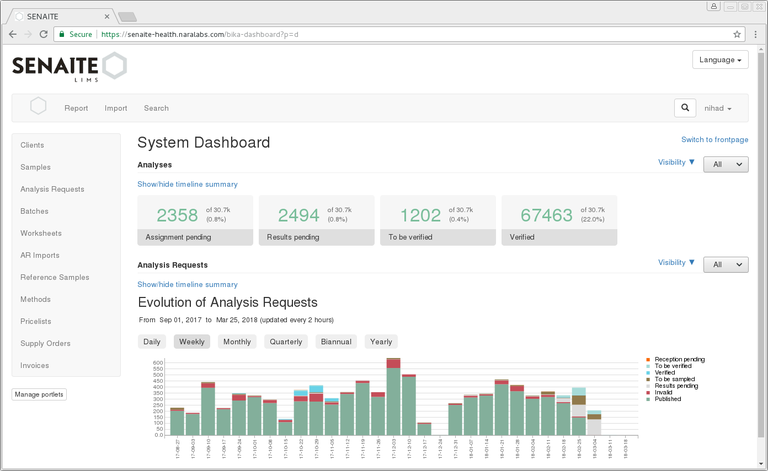
Has had 162 commits made by 16 contributors representing 866 528 lines of code.
It contains management for projects samples and their related data.
Lis is a software solution for improvement of work processes in a biochemical laboratory.
Freelims is a laboratory information management system lims.
Open lims laboratory information management system is an organism independent project management suite for biological projects.
Open lims the open source laboratory information management system open lims open lims.
You can embed statistics from open hub on your site code.
Tracking or remediating known open source vulnerabilities.
Freelims is open source and free.
Create sample types from methods parameters.
It is being developed with the aim to simplify speed up and increase reliability of sample analysis and results issuance.
Windopath ē ssential is the best in klas award winning laboratory information software for anatomic pathology labs.
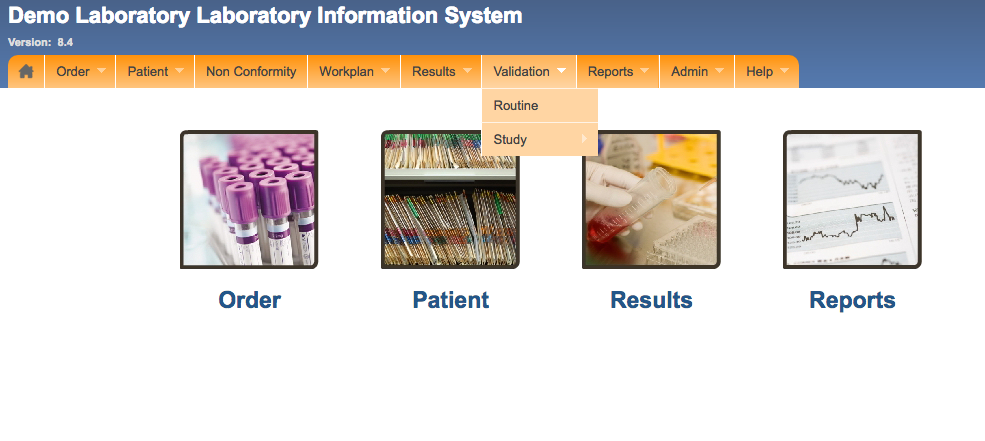
Basic laboratory information s.
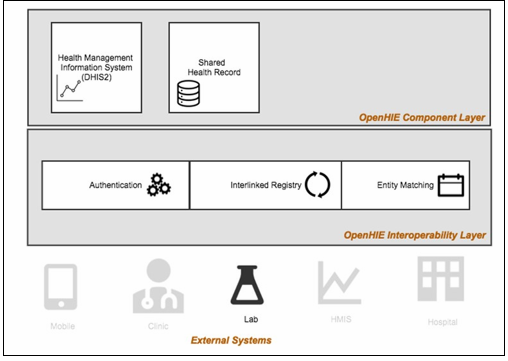
A laboratory information management system lims sometimes referred to as a laboratory information system lis or laboratory management system lms is a software based solution with features that support a modern laboratory s operations.
Is mostly written in php with a low number of.
Key features include but are not limited to workflow and data tracking support flexible architecture and data exchange interfaces which fully support its use in regulated environments.
Fine tune user rights.
Easily generate reports certificates.